Associate Professor Michelle Farrar and colleagues from Sydney Children’s Hospital (Randwick) are conducting a new research project to lean more about what factors are important when making decisions related to treatment for spinal muscular atrophy (SMA). They are seeking the views of parents/carers of people with SMA, health care providers and the wider community. Please find attached more […]
Archive | Clinical RSS feed for this section
Recruitment Request: Understanding patients’ and carers’ decision making in Spinal Muscular Atrophy Care
Early Access Program for Nusinersen
Early Access Program for Nusinersen There has been a lot of activity in the clinical trial space for the treatment of Spinal Muscular Atrophy (SMA) over the last two years, and Nusinersen, has been identified as the first effective drug to treat SMA Type 1. Developed in California, Nusinersen (know in the USA […]
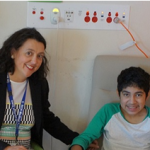
Lifeline for Pompe disease patients
RCH clinicians have worked with the Australian Pompe Association, drug company Genzyme and the Federal government to achieve government-subsidised treatment for late-onset Pompe disease. 12-year-old patient Christian Rivera received his first infusion at the RCH last week. The federal government has listed the only registered treatment for Pompe disease, Myozyme (alglucosidase alfa), on the Life […]

National Disability Insurance Scheme
UPDATE: On Friday 19th August, State Premiers, Territory Chief Ministers and Disability Ministers attended the Council of Australian Governments (COAG) meeting in Canberra, and the NDIS was high on the agenda. Below is the joint media release from the Prime Minister’s office (visit the Every Australian Counts website for more information). Prime Minister – Joint […]
